

 Rosa Mulder1,2 Esther Walton3,4 & Charlotte Cecil1,5,6
Rosa Mulder1,2 Esther Walton3,4 & Charlotte Cecil1,5,6
Follow Esther and Charlotte on Twitter.
Epigenetics can help explain how our genes and environment interact to shape our development. Interest in epigenetics has grown increasingly within the research community, but until now little was known about how epigenetics change over time. We therefore studied changes in our epigenome from birth to late adolescence and created an interactive website inviting other researchers to explore our findings.
What is epigenetics?
The term ‘epigenetics’ refers to the molecular structures around the DNA in our cells, that affect if, when, and how our genes work. Even though nearly every cell in our body contains the exact same copy of DNA, cells can look and function entirely differently. Epigenetics can explain this. For example, every cell in our body has the potential to store fat, but in adipose tissues the cells’ epigenetic structures cause the cells to actually store fat.
Before birth, epigenetics plays a role in the specialization of cells from conception onwards by turning genes ‘on’ and ‘off’. After birth, epigenetics help our body develop even further, and maintain the specialization of our cells. However, the way epigenetics influence how our cells function is not only programmed by our genes, but may also be affected by the environment. Hence, our development and health is shaped by both our genes and our environment. Researchers are therefore trying to measure epigenetic processes to understand the role that epigenetics plays in this process of ‘nurture affecting nature’.

How can we measure epigenetics?
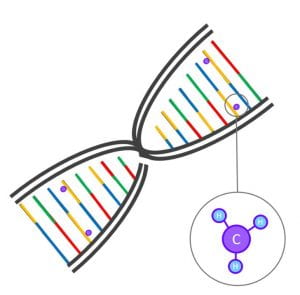
One of the types of molecular structures that can affect gene functioning is ‘DNA methylation’. Here, a small molecule (a methyl group of one carbon atom bonded to three hydrogen atoms; Figure 1) is attached to the DNA sequence. DNA methylation affects the three-dimensional structure of the DNA and can thereby turn it ‘on’ or ‘off’. DNA methylation can now easily be measured in the lab with the help of micro-chips; very small chips that can detect hundreds of thousands of methylation sites in the genome at a time, from just a small droplet of blood. Such chips are now used in large epidemiological cohorts such as ALSPAC to measure the level of DNA methylation for each of these sites. In epigenome-wide associations studies (EWASs), researchers study the associations between each of these methylation sites and a trait, such as prenatal smoking, BMI, or stress.
How does DNA methylation change throughout development?
Until recently, EWASs have mainly been cross-sectional, studying DNA methylation only at one time-point. So, even though research indicates that epigenetics is important in postnatal development, we do not know how true this is for DNA methylation sites measured with these epigenome-wide arrays. Studying a mechanism that supposedly changes over time without knowing how it changes can be problematic: say that we find an association between smoking during pregnancy and DNA methylation at birth, can we still expect this association to be there at a later age? To fully interpret EWAS findings, and to compare research findings between different studies, we need a full understanding of how DNA methylation changes throughout development.
We therefore set out to study DNA methylation from birth to late adolescence, using DNA methylation measured in blood from the participants of ALSPAC in the UK, as well as from participants from another large cohort, the Generation R Study in the Netherlands.
We studied the change in levels of DNA methylation over time as well as variation in this change between individuals. If DNA methylation is indeed mainly linked to the basic developmental stages we go through as we grow up, we would expect methylation changes to be largely consistent between individuals. However, if DNA methylation is affected more by the different environments we live in, and individual health profiles, we would expect a proportion of sites to change differently for different individuals.
Between ALSPAC and Generation R, we created a unique dataset containing over 5,000 samples from about 2,500 participants with DNA methylation measurements at almost half a million methylation sites measured repeatedly at birth, 6 years, 10 years, and at 17 years. With various statistical models we studied different trajectories of change in DNA methylation.
We found change in DNA methylation at just over half of the sites (see for an example Figure 2a). At about a quarter of sites, DNA methylation changed at a different rate for different individuals (Figure 2b). We further saw that sometimes change only happened in a specific time period; for example, only in between birth and the age of 6 years after which DNA methylation remained stable (Figure 2c), and that sometimes differences in the rate of change only started from the age of 9 years (Figure 2d). Last, for less than 1% of the sites on the chromosomes tested (we did exclude the sex chromosomes), we saw that DNA methylation changed differently for boys and girls (Figure 2e).

How can we use these findings in future research?
These results show that there are sites in the genome for that show change in DNA methylation that is consistent between individuals, as well as sites that change at a different rate for different individuals. We have published the trajectories of change for each methylation site on a publicly available website. This makes it easier for other researchers to find sites that are developmentally important and may be of relevance for health and disease. For example, a methylation site previously associated with prenatal smoking, remained stable over time (Figure 1f), indicating that prenatal influences of smoking may be long-lasting, at least up to adolescence. In the future, we hope to associate traits, such as stress and BMI, to these longitudinal changes, to further our understanding of the developmental nature of DNA methylation and the associated biological pathways leading to health and disease.
1Department of Child and Adolescent Psychiatry/Psychology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands
2 Department of Child and Adolescent Psychiatry/Psychology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands
3 MRC Integrative Epidemiology Unit, Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK
4 Department of Psychology, University of Bath, Bath, UK
5 Department of Epidemiology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands
6 Department of Psychology, Institute of Psychology, Psychiatry & Neuroscience, King’s College London, London, UK
Further reading
Mulder, R. H., Neumann, A. H., Cecil, C. A., Walton, E., Houtepen, L. C., Simpkin, A. J., … & Jaddoe, V. W. (2020). Epigenome-wide change and variation in DNA methylation from birth to late adolescence. bioRxiv. (preprint)
Epidelta project website: http://epidelta.mrcieu.ac.uk/
